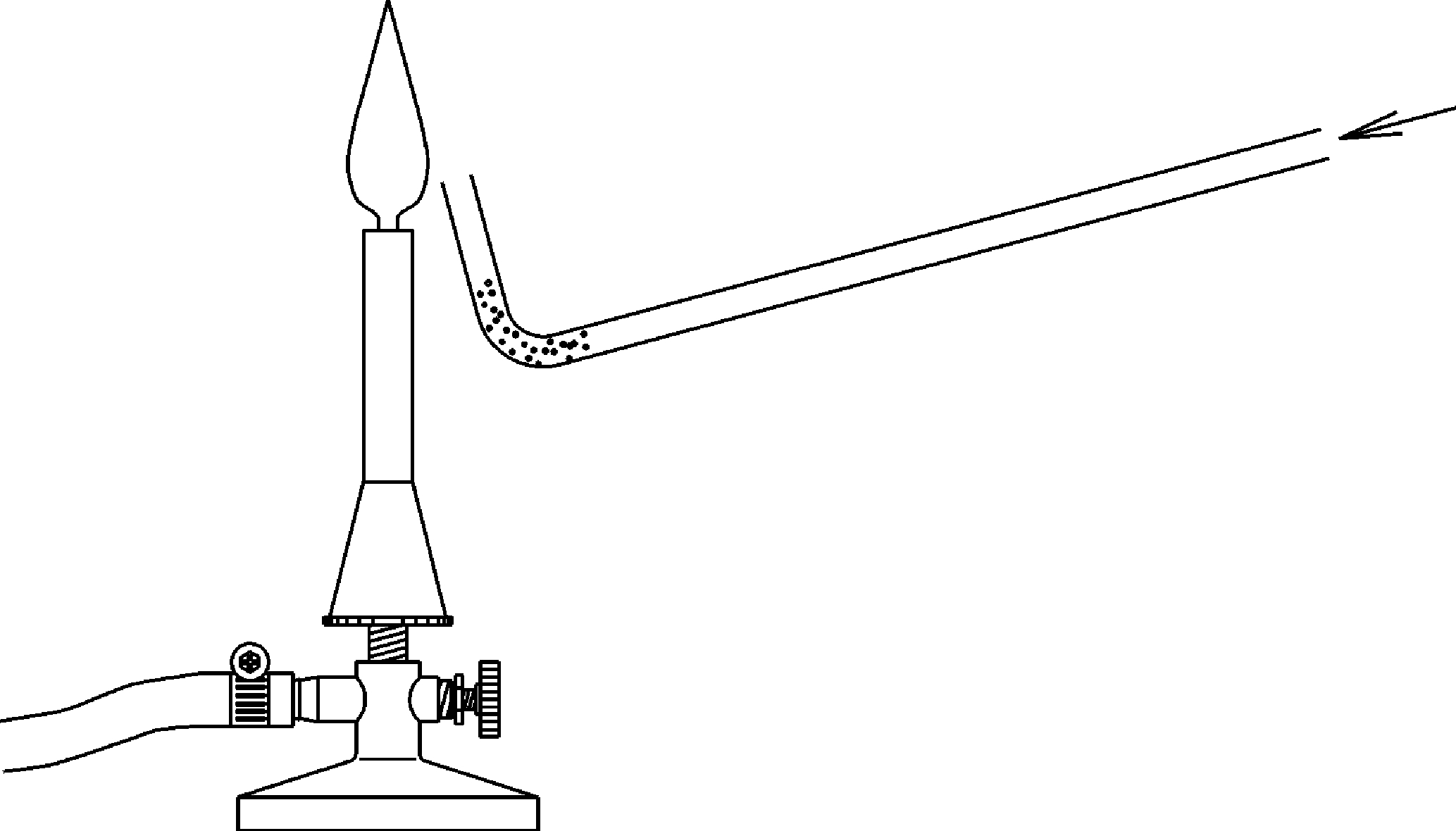
Principle
This experiment shows that the combustion rate depends on the degree of dispersion and the mixing with oxygen (or the oxidizing agent). Students observe that metals such as aluminum and magnesium are non-flammable in compact form or when they burn , they burn relatively slowly. However, in pulverized form this metals burn rapidly. The highest combustion rates can be reached with mixtures of combustible gases with air or with oxygen.
Learning objectives
- Combustion reaction
- Combustion rates of different metals