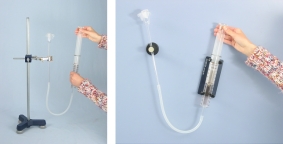
Principle
If the volume of an enclosed gas is reduced, then the pressure of the gas increases, if the volume is increased, then the pressure decreases. The pressure difference to air pressure can be so great that the diaphragm bursts with a loud bang.Benefits
- Simple and clear setup
- Achievement of an elemental learning objective with little effort