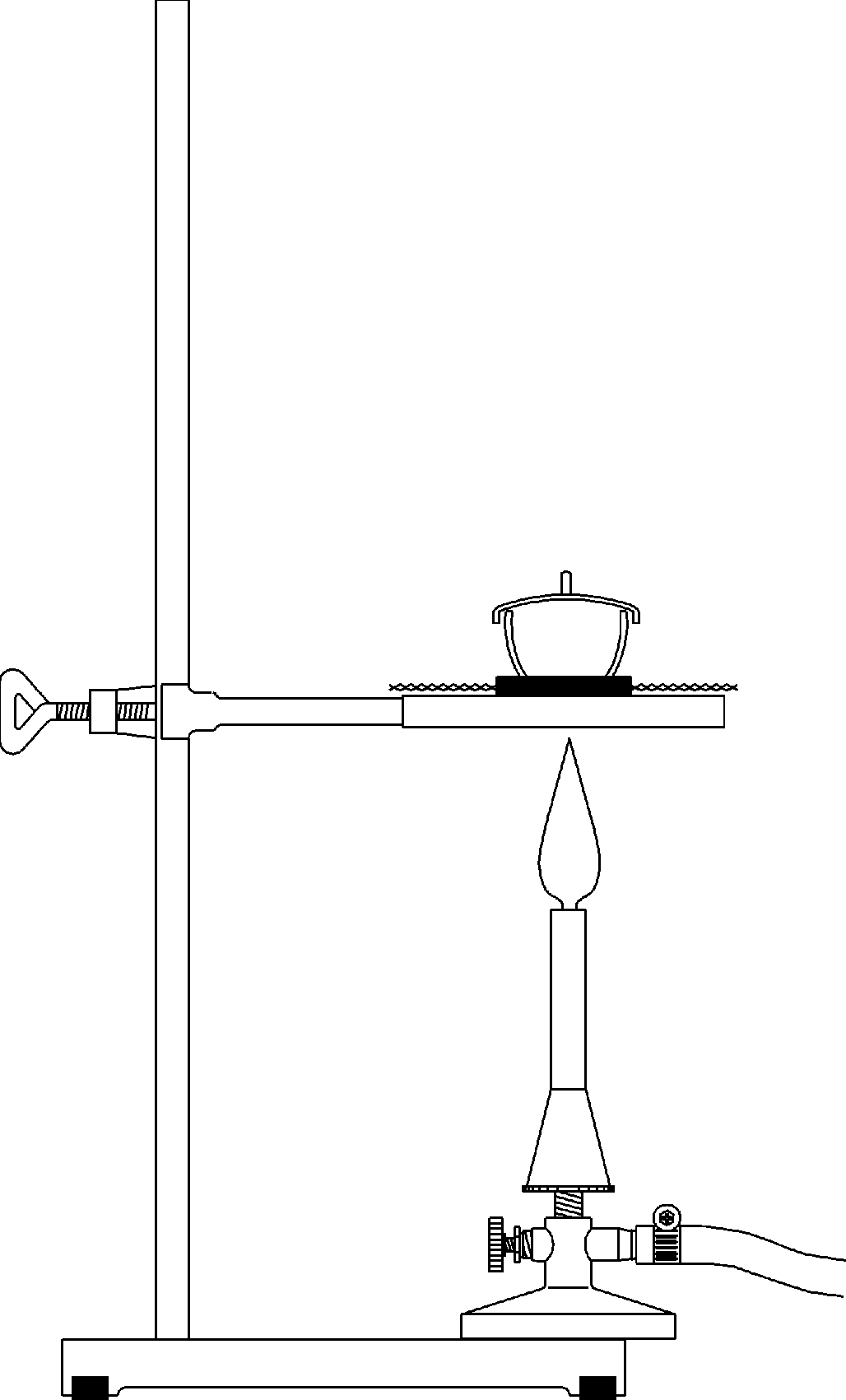
Principle
In the first part of the experiment, "synthesis as a chemical reaction" is examined in more detail and the behaviour of copper sheet in sulphur vapour is investigated. To do this, a piece of sulphur is heated in a test tube and then a strip of copper sheet is held in the steam. This causes copper and sulfur to combine, releasing energy to form a new substance.
In the second part of the experiment, the analysis is examined using the example of the reduction (reduction as the reverse process of oxidation) of copper oxide to copper. It can be seen that many oxides (formed by a thermal reaction of atmospheric oxygen with an element) cannot be thermally decomposed in a simple way, but can be returned to the corresponding element with the aid of reducing agents (e.g. carbon).