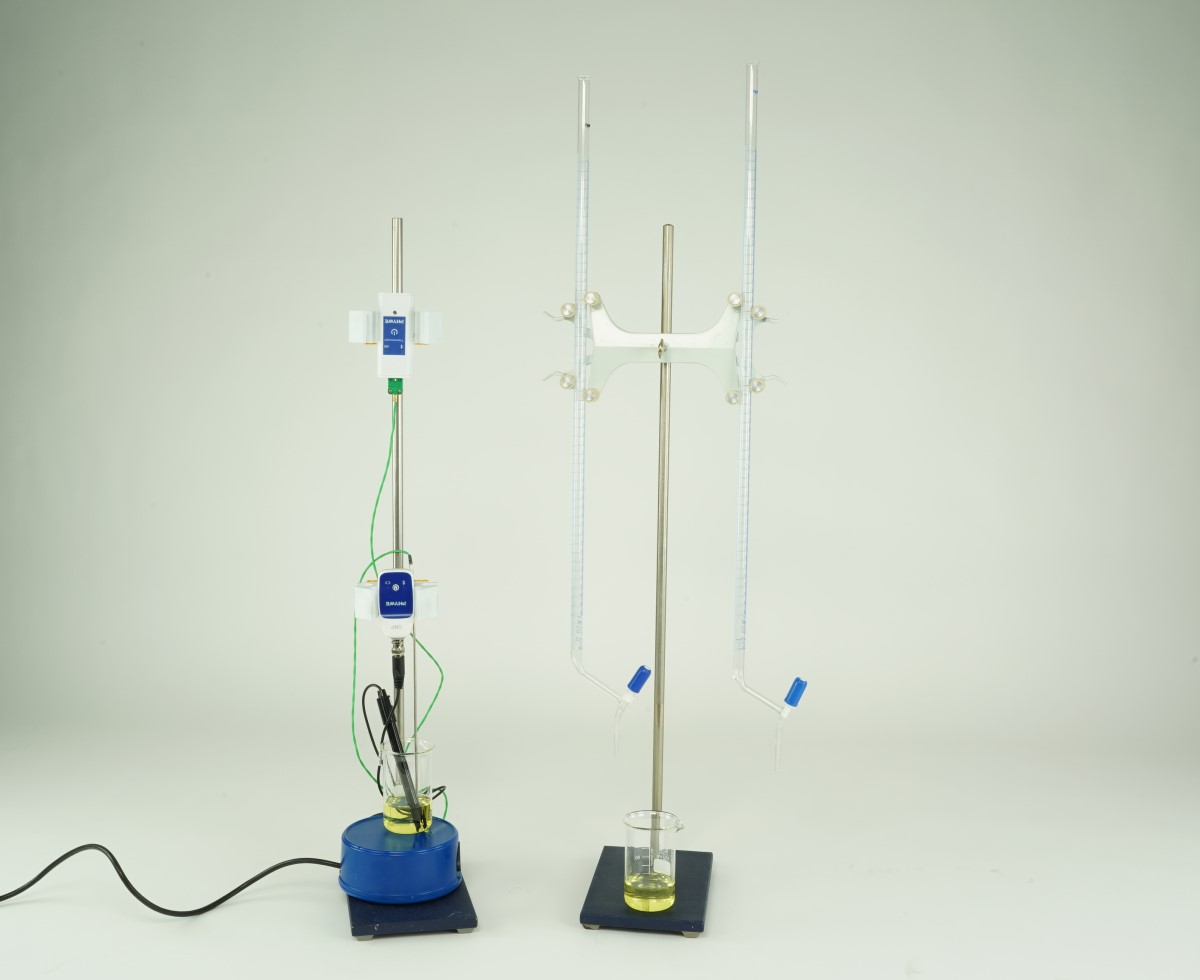
Principle
The Nernst equation states how the electrical potential of an electrode in contact with an ionic solution depends on the concentrations (more precisely, activities) of these ions. The equation can be verified experimentally by using an electrochemical cell consisting of an inert indicator electrode coupled to a suitable reference electrode (here in the form of an ORP combination electrode).
Benefits
- Relevant both for chemists and for physicists
- Essential introductory experiment for studying battery technology
Tasks
Measure the potential of a platinum electrode with our ORP electrode immersed in solutions of known concentrations of the iron(ll) and iron(lll) complex ions [Fe(CN)6]4 - and [Fe(CN)6]3-.
Learning objectives
- Electrode potentials and their concentration dependence
- Redox electrodes
- Electrochemical cells
Necessary accessories
- Analytical balance 120g/0.1mg
Software included. Computer not provided.
Necessary accessories
You cannot use the article without these accessories!